Results
The manufacturing costs of insect meal production depend to a large extent on the cost of raw materials. As an approach to optimize the costs of the production process, low-cost raw material sources based on residues have been investigated as a substrate for insect production. This included the investigation of substrate suitability, characterization of material properties and concept development for raw material handling.
Procedure and methodology
Basics and experimental setup
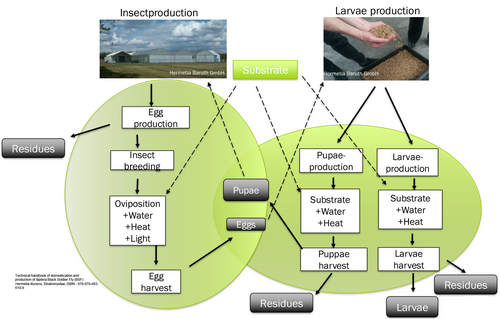
The entire process of hermetia production can be subdivided into two production stages, whereby the first stage of insect production focuses on insect recovery and the second stage of larval production involves the rearing of young larvae. Figure 1 shows a schematic representation of the hermetia production process.
During insect production, the eggs of the hermetia fly are collected, which are used in larval production for breeding insect larvae and pupae. Some of the larvae develop into hermetia pupae, which grow into sexually mature hermetia flies during insect production and lay new hermetia eggs. The remaining hermetic larvae, which are not used for insect production, are removed from the larval production before pupation and processed into hermetic meal. The use of cost-effective feed materials is of particular economic importance in the production stage of larval production, as large quantities of substrate are required for the rearing of young larvae. On a laboratory scale, different feed alternatives have been investigated in detail, especially in the field of larval production.

Hermetia Baruth GmbH provided young larvae for laboratory-scale feeding trials. The young larvae were delivered to the DBFZ laboratory at an average age of 9-12 days after egg deposition. At this time the young larvae had an average weight of approx. 2.4mg and an average size of 2 mm. The growth of the young larvae was tested on different substrates in parallel feeding experiments on a scale of approx. 250ml. For this purpose, the young larvae and the test substrate were each weighed in as a triple batch in different mixing ratios and tempered over the entire test period (see Figure 3). The larval growth and the larval end weight were documented over a trial period of 13 - 17 days. The experiments were performed until the weight of the larvae remained constant. After reaching the abort criterion, the sample preparations were sieved and residual materials and larvae were examined individually. Material analyses were carried out on the adult larvae and the input and residual materials. The growth rate of the larvae could be calculated and the overall efficiency of the feed conversion of the investigated substrates could be evaluated.
Substrates investigated
The following raw materials have so far been investigated as potential feed for hermetic larval breeding:
Residual and waste materials
- dry chicken excrement
- Fermentation residue from agricultural biogas plant
- Fermentation residue from industrial biogas plant
- Aquatic plant Elodea Nutallii
- Aquatic plant silage Elodea Nutallii
- Aquatic plant Myophyllum
- Aquatic plant Lemna Minor
- Aquatic plant Nymphaea alba
- Corn silage feed residues from dairy cow husbandry
- Feed remains from insect breeding
Residues or by-products with livestock feed quality
- brewer's grains
- Dried pressed stillage (DDGS) from bioethanol production
- Grain stillage liquid silage
- coffee residues
The substrate selection can be divided into two groups. The residues of cereal stillage, brewer's grains and coffee residues belong to the feed materials that are legally harmless for animal feed and may be used for the economic production of Hermetia according to current legal requirements. According to EU Regulation 1069/2009 Animal by-products (Article 3 point 6), the black soldier fly is defined as a farm animal. Thus, in Europe at present only animal feeds may be used as input materials for hermetia production. Regulation 999/2001 BSE/TSE states that no processed animal protein (PAP) may be fed to farm animals. In 2001, only the exception fishmeal was allowed. Since 1 July 2017, protein meal from seven insect species may be fed to fish in aquaculture (farm animals). The regulation is an annex to 999/2001 and has the number 2017/893. The issue of insects is part of other relevant regulations such as Regulation 767/2009 - Marketing and use of feed and Regulation 68/2013 - Catalogue of feed materials.
Investigated process conditions
In parallel feeding experiments with different substrate samples, the following influencing factors on the growth behaviour of hermetic larvae were investigated in more detail:
- Process temperature
- Temperature of the substrate at the beginning of the test
- Feed concentration and water content of the substrate material
- Drying behaviour of the substrate during the feeding experiment